LIVESTOCK
D-Bloat Liquid: Powerful Relief for Frothy & Gaseous Bloat in Livestock
₹85 – ₹140Price range: ₹85 through ₹140
Select options
This product has multiple variants. The options may be chosen on the product page
VetoFeed MV: Premium Chelated Vitamin & Mineral Supplement for Livestock, Poultry & Fish
₹310 – ₹4,300Price range: ₹310 through ₹4,300
Select options
This product has multiple variants. The options may be chosen on the product page
CalciLiv Oral Glycine CHELATED – Ultimate Calcium Supplement for Dairy Animals
₹210 – ₹3,800Price range: ₹210 through ₹3,800
Select options
This product has multiple variants. The options may be chosen on the product page
ReproMIN Dry – The Reproduction Formula for Dairy Animals
₹435 – ₹3,900Price range: ₹435 through ₹3,900
Select options
This product has multiple variants. The options may be chosen on the product page
































































































 Sale - Buy 3 at 899 or Buy 6 at 1599
Sale - Buy 3 at 899 or Buy 6 at 1599




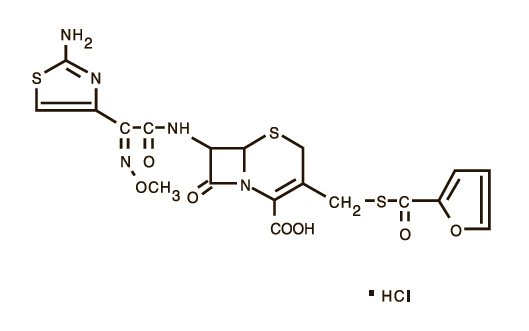












































Reviews
Clear filtersThere are no reviews yet.